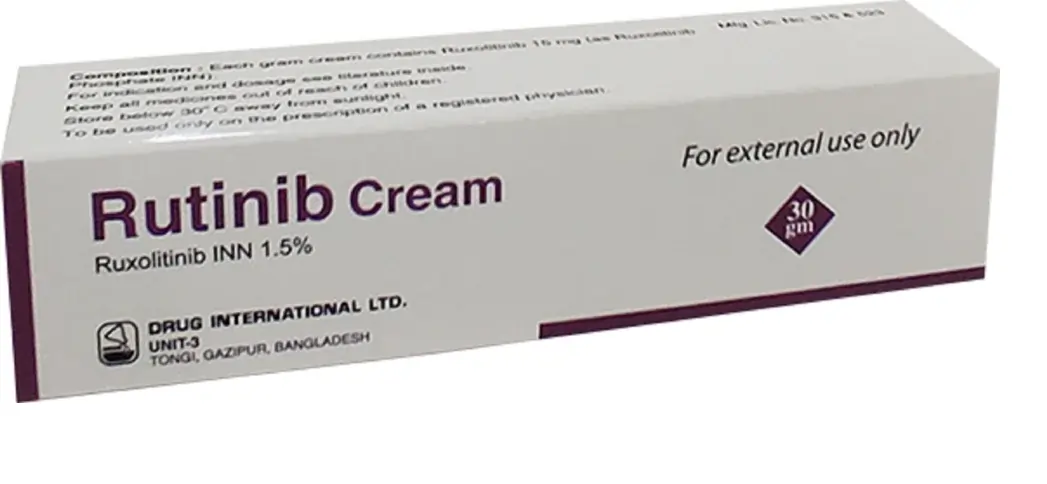
About Rutinib Ruxolitinib Cream
Prescription medication Rutinib (Ruxolitinib Cream), also known as generic OPZELURA, is utilized to manage atopic dermatitis, a mild to moderate skin condition, in non-immunocompromised individuals aged 12 and above. This condition is not deemed to be treatable with topical prescription therapies or when such therapies are contraindicated. Ruxolitinib Cream is additionally employed in the management of nonsegmental vitiligo, a form of vitiligo that affects infants and adults aged 12 years and older. Eczema is an allergic disorder characterized by pruritus, pruritus, and pruritus of the epidermis. By inhibiting the skin’s immune system, Ruxolitinib Cream 1.5 reduces the severity of allergic reactions and alleviates dermatitis.
The Dosage
To apply Rutinib 1.5% cream, coat the affected area with a thin layer of cream. Its use should be discontinued once the observed signs and symptoms subside, including erythema, rash, and pruritus. You should reevaluate the situation after 56 days if it has not improved.
The Uses of The Rutinib Ruxolitinib Cream
Particular skin conditions, including vitiligo and eczema (atopic dermatitis), are managed with ruxolitinib cream. Eczema is an allergic disorder characterized by pruritus, pruritus, and pruritus of the epidermis. Vitiligo is a condition in which the pigment-producing skin cells are attacked by the immune system. By inhibiting the skin’s immune system, this medication may be able to alleviate the symptoms of the skin conditions. Rutinib 1.5 is a member of the medicinal class referred to as kinase inhibitors.
Ruxolitinib 1.5% Topical Cream Usage Instructions
It is advisable to consult the Medication Guide supplied by your pharmacist prior to initiating Ruxolitinib 1.5% treatment and at each refill time. If you have any inquiries, consult your physician or chemist.
Water and soap should be used to cleanse the hands prior to administering this medication. Typically twice daily, apply a fine layer to the affected areas of skin as directed by your physician. Apply the medication to the epidermis in a thorough and gentle massage. Unless your hands are being treated, wash your hands after using this product. Follow the instructions of your physician regarding the application of an emollient subsequent to taking this medication.
Only apply this product to the epidermis. It is advised to refrain from ingesting this medication through the eyes, nostrils, or mouth. If the medication does enter those areas, thoroughly cleanse them with water. This medication should not be applied to exposed wounds or infected areas. Protect the treated area from moisture and plastic or impermeable bandages, unless otherwise instructed by your physician.
Take this medication precisely as prescribed. In the case of eczema treatment, your physician may advise you to discontinue the use of this product once the condition has resolved, but to resume application should symptoms resurface. Consult your physician for further information. Unless otherwise prescribed, do not exceed 60 grams of this medication per week or 100 grams every two weeks.
Dermatitis is an umbrella term that encompasses a variety of skin irritations. Dermatitis causes an awful amount of pain and distress that we must endure. In contrast, dermatitis is entirely curable through the use of Rutinib moisturizer. Drugcen is a reliable source for purchasing critical products such as Rutinib cream 1.5%.
Drugcen offers affordable anti-cancer medications on a global scale. Please contact us if you have any inquiries or wish to learn more about our products.
Advice From An Expert on Ruxolitinib:
- It is preferred to take Rutinib cream 1.5 daily at the same time, with or without meals.
- Headaches and dizziness may result. Drive or engage in any activity requiring mental concentration until you have determined how it affects you.
- It is advised to utilize a dependable contraceptive method in order to avoid pregnancy while on this medication.
- While you are on this medication, your physician may order routine blood tests to monitor your lipid levels, liver and kidney function, and blood cell counts.
- Immediately consult your physician if you develop any symptoms of an infection, including fever, hoarse throat, or rash.
- Prior to discontinuing Ruxolitinib, consult your physician.
Thorough Precautions and Caution:
Drug Cautions
If you are pregnant or breastfeeding, or if you have an allergy to any of its components, you should not take RUXOLITINIB. Notify the physician of any of the following: skin cancer, HIV or AIDS, anemia, blood clots, heart attack or stroke, shingles, cardiac problems, high cholesterol, low blood count, tuberculosis, hepatitis B, kidney or liver problems, skin cancer, or any of the aforementioned conditions. It is advised to promptly seek medical attention if one encounters the following symptoms of a serious brain infection: fever, chills, infections (including fever and chills), tuberculosis (characterized by persistent blood-tinged sputum, night sweats, weight loss, and confusion or difficulty thinking, impaired vision or weakness, difficulty speaking, weakness, blurred vision, or loss of vision), or tuberculosis (causing unexpected bleeding or bruising, shortness of breath, frequent infections, unusual fatigue or pale skin). Inform your physician if you are concurrently using any other medications, herbal remedies, or supplements.
Interactions with Medications:
Drug Interactions Among Medications: Please ensure that your healthcare provider is aware of any concurrent medications you may be taking, including antifungal agents (e.g., voriconazole, posaconazole, itraconazole, posaconazole, fluconazole, or voriconazole), antibiotics (e.g., clarithromycin, telithromycin, ciprofloxacin, erythromycin), antiviral drugs (e.g., amprenavir, atazanavir, indinavir, lopinavir/ritonavir, nelfinavir, ritonavir, saquinavir), hepatit
Food Drug Interactions:
The consumption of grapefruit juice has the potential to elevate blood concentrations of Rutinib 1.5. Grapefruit and grapefruit juice should not be consumed while taking unless otherwise directed by a physician.
Anxiety Drug Interactions:
Cancer, cardiac problems, thrombosis (blood clots), hepatitis B infection, tuberculosis, infections, high lipid levels, lung problems, or tuberculosis should all be disclosed to the physician.
FAQs:
1. How exactly does Ruxolitinib function?
In order to treat myelofibrosis and polycythaemia vera, RUXOLITINIB 1.5% inhibits the signals that promote the multiplication of cancer cells. It aids in the treatment of graft-versus-host disease (GVHD) through the inhibition of GVHD-causing cell signals.
2. Is Ruxolitinib associated with the development of infections?
RUXOLITINIB 1.5% has the potential to impair hepatic function and elevate the susceptibility to severe infections. If you develop symptoms of infection including fever, shivers, sweating, sore throat, cough, sore throat, frequent/urgent/painful urination, or cough, consult a physician.
3. May I incorporate additional medications while on Ruxolitinib treatment?
While on treatment with RUXOLITINIB 1.5%, do not begin taking any new medications without first consulting your doctor, including prescription, over-the-counter, herbal, or dietary supplements.
4. Does Ruxolitinib elevate the susceptibility to tuberculosis?
RUXOLITINIB has the potential to elevate the susceptibility to tuberculosis (TB), especially in individuals who are asymptomatic but already contaminated with the disease. Please notify your healthcare provider if you have ever been diagnosed with tuberculosis or if you develop any of the following symptoms: weight loss, fatigue or lethargy, loss of appetite, chills, fever, or night sweats.
5. What is the duration of action of Rutinib Cream 30 Ruxolitinib?
Patients undergoing treatment with Rutinib Cream 1.5 may apply the cream to the affected areas twice daily, encompassing a maximum of 10 percent of the body’s surface area. Incyte states that effective results may not be visible for individuals with vitiligo for at least 24 weeks.